
客户服务热线
400-0470-600

微信
13903605303

|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HaiGene Bst dU5.0 DNA聚合酶是HaiGene于2025年底推出的创新型Bst DNA聚合酶。该酶融合了Bst2.0(dUTP渗入能力)、Bst3.0(快速扩增、抗杂质)、Bst4.2(70℃高温反应)优势于一身,与高性能的Bst XT等聚合酶相比,该酶还可完全使用dUTP(100%)来代替反应体系中的dTTP。100%dUTP的使用能力,对于LAMP的防污染至关重要。此外,该酶不仅通用于DNA或RNA的LAMP扩增,而且全系产品均为热启动版本。 性能特点:(1)Bst dU5.0 DNA聚合酶包含热启动Aptamer,其确保在<30℃时,酶活封闭效率>90%,在>60℃时1min内完全释放酶活。该特性利于室温建立反应体系,并大幅降低了低温条件下的非特异扩增;(2)在65-70℃条件下,均可有效进行LAMP扩增;(3)相比于Bst2.0 dUTP的使用比例仅50%(这无法满足LAMP的防污染要求),Bst dU5.0无需使用dTTP,可完全替换成dUTP,速度几乎无下降。在配合热敏UDG的条件下,可有效的防止气溶胶引起的污染。 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
性能展示 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
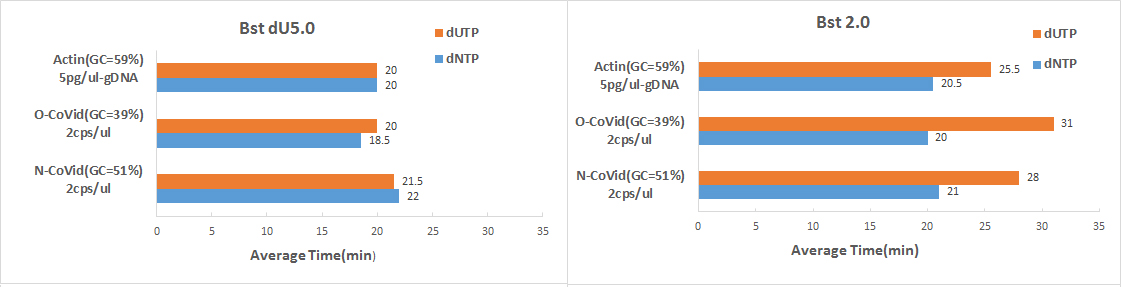
| 1、dNTP和dUTP使用速度比较 Bst 2.0与Bst dU5.0在使用100%dUTP核苷酸底物时,LAMP扩增低丰度模板的情况比较。结果显示:Bst dU5.0在使用100%dUTP时,表现出与dNTP一致的扩增速度。而Bst2.0在使用全dUTP时,出现不同程度的扩增滞后(可能与扩增区域GC含量有关)。 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
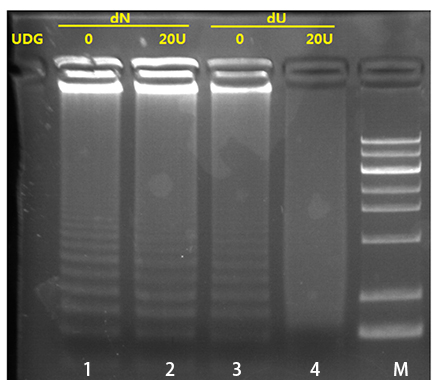
| 2、UDG对Bst dU5.0聚合酶的100%dU 扩增产物的消化能力测试 泳道1:dNTP扩增产物,无UDG消化;泳道2:dNTP扩增产物,20U的UDG消化;泳道3:100%dUTP扩增产物,无UDG消化;泳道4:100%dUTP扩增产物,20U的UDG消化。结果显示:高分子量的dU-LAMP产物均被UDG酶有效的切割。在后续的扩增中,该产物不能再被扩增,从而有效的避免气溶胶污染。 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
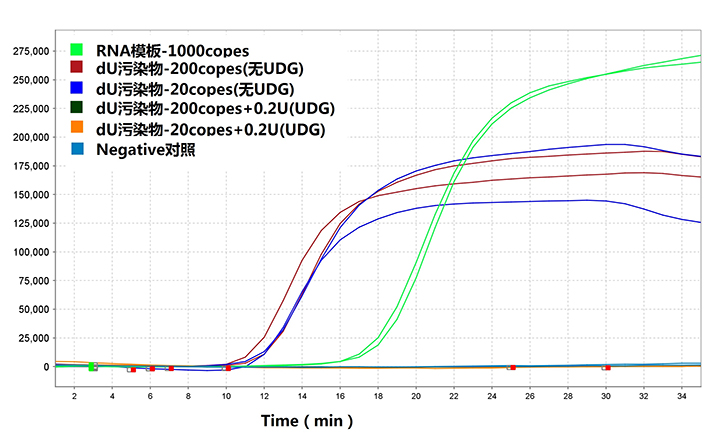
| 3、Bst dU5.0 DNA聚合酶荧光LAMP抗污染能力测试 在20μl的LAMP反应体系中加入污染物,即造成远高于检测模板的扩增(蓝线和红线),由图可见20cps-污染物(蓝线)快于1000cps-RNA(绿线)的检测。在反应体系中加入终浓度10mU/ul的热敏UDG后,污染物引起的假阳性消除(黑线和橙线)。结果表明:采用100%dU作为底物进行LAMP扩增,并联合热敏UDG可有效减少LAMP假阳性扩增。值得注意的是,在HaiGene的其它测试中加入20%的dTTP后,假阳性扩增并不能有效消除。这表明在LAMP扩曾中,为防止假阳性扩增,采用100%dUTP作为底物的必要性,HaiGene确保Bst dU5.0系列试剂为100%dU底物。因此建议实验室始终采用dUTP为底物进行LAMP扩增,一旦发生污染,可通过加入热敏UDG进行消除。而采用dNTP扩增导致气溶胶污染后,整个实验室将面临数周以上的停摆风险。 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
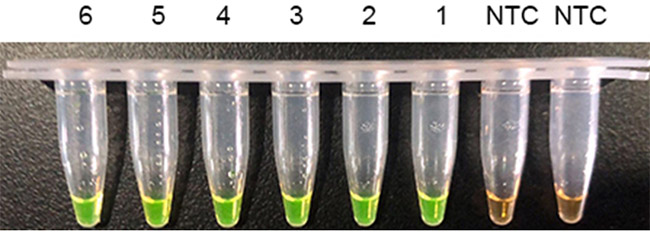
| 4、以Bst dU5.0 Basic试剂搭配 OG 橙绿变色反应管(A3828-21) 以2019-nCoV体外转录RNA为模板,1-6分别为1、10、100、1000、10e4和10e6Copy,NTC为ddH2O为模板。上图为加样结束反应起始前,下图为70℃反应30min后。 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
应用LAMP产品文献 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1. Rapid Visual Detection of Elite Erect Panicle Dense and Erect Panicle 1 Allele for Marker-Assisted Improvement in Rice (Oryza sativa L.) Using the Loop-Mediated Isothermal Amplification Method. Curr Issues Mol Biol. 2024 Jan 4;46(1):498–512. 2. Rapid Visual Detection of High Nitrogen-Use Efficiency Gene OsGRF4 in Rice (Oryza sativa L.) Using Loop-Mediated Isothermal Amplification Method. Genes (Basel). 2023 Sep 23;14(10):1850. 3. Rapid and visual identification of HIV-1 using reverse transcription loop-mediated isothermal amplification integrated with a gold nanoparticle-based lateral flow assay platform. Front Microbiol. 2023 Jul 12;14:1230533. 4. Rapid Detection of Streptococcus mutans Using an Integrated Microfluidic System with Loop-Mediated Isothermal Amplification. J Microbiol Biotechnol. 2023 May 19;33(8):1101–1110. 5. Rapid point-of-care detection of BK virus in urine by an HFman probe-based loop-mediated isothermal amplification assay and a finger-driven microfluidic chip.PeerJ. 2023 Mar 8;11:e14943. 6. Rapid detection of monkeypox virus and monkey B virus by a multiplex loop-mediated isothermal amplification assay. J Infect. 2023 Feb 13;86(4):e114–e116. 7. SMART: A Swing-Assisted Multiplexed Analyzer for Point-of-Care Respiratory Tract Infection Testing. Biosensors (Basel). 2023 Feb 4;13(2):228. 8. An HFman Probe-Based Multiplex Reverse Transcription Loop-Mediated Isothermal Amplification Assay for Simultaneous Detection of Hantaan and Seoul Viruses. Diagnostics (Basel). 2022 Aug 10;12(8):1925. 9. Real-Time Detection of LAMP Products of African Swine Fever Virus Using Fluorescence and Surface Plasmon Resonance Method. Biosensors (Basel). 2022 Apr 3;12(4):213. 10.Multiplex, Real-Time, Point-of-care RT-LAMP for SARS-CoV-2 Detection Using the HFman Probe.ACS Sens. 2022 Feb 22;7(3):730–739. 11.Deep‐dLAMP: Deep Learning‐Enabled Polydisperse Emulsion‐Based Digital Loop‐Mediated Isothermal Amplification. Adv Sci (Weinh). 2022 Jan 24;9(9):2105450. 12.Development of a TaqMan loop-mediated isothermal amplification assay for the rapid detection of pigeon paramyxovirus type 1. Arch Virol. 2021 Mar 23;166(6):1599–1605. 13.Rapid, visual, label-based biosensor platform for identification of hepatitis C virus in clinical applications. BMC Microbiol. 2024 Feb 28;24:68. 14.A novel extraction-free dual HiFi-LAMP assay for detection of methicillin-sensitive and methicillin-resistant Staphylococcus aureus.Microbiol Spectr. 2024 Feb 20;12(4):e04133-23. 15.A duplex HiFi-LAMP assay for screening of two novel human circoviruses HCirV-1 and HCirV-2. BMC Infect Dis. 2024 Dec 5;24:1388. 16.Point-of-care test of blood Plasmodium RNA within a Pasteur pipette using a novel isothermal amplification without nucleic acid purification. Infect Dis Poverty. 2024 Oct 31;13:80. 17.Rapid detection of human influenza A viruses by HFman probe-based loop-mediated isothermal amplification assays. Heliyon. 2023 Oct 25;9(11):e21591. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||